Core Technology
The combustion synthesis technology (CS), also known as self-propagating high-temperature synthesis (SHS), is a method for preparing high-temperature inorganic materials in recent years. It is based on a nitriding reaction, which is a strongly exothermic reaction that meets the conditions required for self-propagating high-temperature synthesis. The preparation of silicon nitride powder using this method involves a synthetic reaction between the elemental forms of silicon and nitrogen. This reaction does not require temperature control; once ignited, it does not need any further energy supply, as the reaction is fundamentally based on a strong exothermic reaction that allows it to continue in the form of a reaction wave.
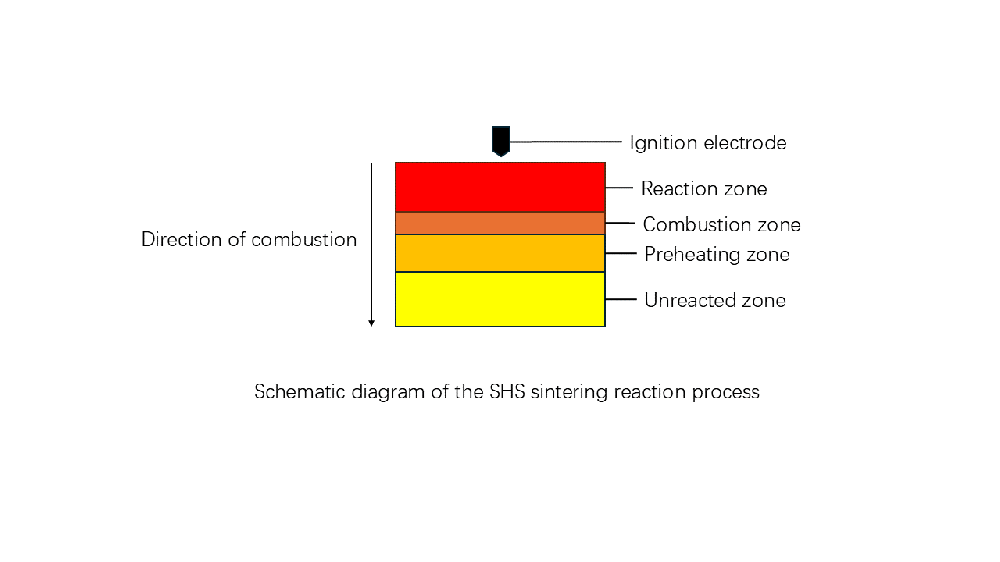
The self-propagating synthesis process is not easy to control, and the reactions or the propagation of combustion waves triggered by burning occur quite rapidly, generally at a rate of 0.1 to 20.0 cm/s, with a maximum of up to 25.0 cm/s. The temperature of the combustion wave or the reaction temperature is usually above 2100 to 3500 K, reaching as high as 5000 K. Compared to traditional preparation processes, the self-propagating method reduces the process steps, shortens the flow, and simplifies the procedure without the need for additional energy consumption. The high temperatures generated as the combustion wave passes through the sample can eliminate easily volatile impurities, thereby increasing the purity of the product. Additionally, the combustion process features a significant thermal gradient and a rapid condensation rate, which may lead to the formation of complex phases, facilitating the transformation from certain raw materials to another product.
The production of silicon nitride powder using the self-propagating high-temperature synthesis (SHS) method typically contains multiple phases, generally the α phase and β phase. The SHS method for synthesizing Si3N4 powder features a simple production process and equipment, rapid reaction speed, and high purity of the synthesized silicon nitride powder; however, controlling the temperature during the reaction is challenging, often resulting in a higher generation of β-Si3N4 due to excessive reaction temperatures. Although large-scale production has been achieved, controlling the phase composition remains difficult. The use of ammonium salts such as NH4Cl as additives can appropriately reduce the nitrogen pressure required in SHS (generally greater than 10 MPa). Researchers believe that the role of these additives as diluents can increase the contact opportunities between the raw materials and nitrogen, thereby promoting the nitridation reaction and enhancing the content of α-Si3N4.

 19194976688
19194976688




